Background
The prevalence of HIV-associated neurocognitive disorders (HAND) remains high among HIV-1 infected patients, despite significant advances in antiretroviral therapy (HAART). HAND is characterized by clinical symptoms such as motor and attention deficits, impaired learning and memory, and in severe cases, dementia. The HIV-1 envelope protein gp120 is believed to have a crucial role in the development of these disorders HIV-1-related neurocognitive dysfunction is strongly linked to the host inflammatory regulator α7 nicotinic acetylcholine receptor (A7R) and the signaling of highly reactive oxygen species (ROS). A7R plays a crucial role in regulating calcium ion exchange in nerve cells, immune cells, and endothelial cells. In the brain, antioxidants are present to control and prevent the formation of harmful ROS, which further contribute to the severity of neurodegeneration. Assessing ROS can help predict oxidative tissue damage, including modifications in proteins, lipids, or DNA. ROS tend to accumulate in the small and large intestines, leading to oxidative stress and affecting cognitive function. The prebiotic mangan oligosaccharide (MOS) has been reported to reshape the gut microbiome and increase short-chain fatty acid (SCFAs) production. This, in turn, alters neuronal redox status through the gut-brain axis. There may be a critical role for HIV-1 gp120 (Gp120) protein in these disorders. The microbiota-gut-brain (MGB) axis is bidirectional, and gut microbiota influence brain disorders including HAND, which could be targeted by nutritional interventions.
Methods:
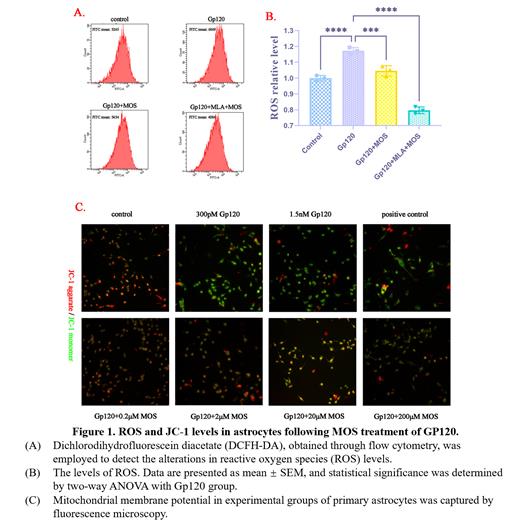
The HIV-1 gp120 transgenic mice (Gp120 transgenic mice), and wild-type (WT) mice were randomly divided into 4 groups (9 months old, n=6 per group): WT group, WT+MOS group, Gp120 transgenic group, Gp120 transgenic + MOS group. All MOS group mice were administered the prebiotic MOS (0.12% w/v in the drinking water).The drinking water added MOS or was replaced twice a week for 8 weeks and recorded water intake. In the in vitro cell experiment, the cortex of neonatal Sprague-Dawley (SD) rats (1-3 days) was dissected to prepare astrocytes. Cells were identified using Cy3-conjugated GFAP immunostaining. Cultured astrocytes were treated with 300 pM Gp120 protein for 24 hours, and then 0.2, 2, 20 or 200 μM MOS was applied for 24 hours, and different proteins level of expression were detected by Western blot. The ROS level were then analyzed on FACS Calibur-Tangerine flow cytometry in 502 nm. The alteration in mitochondrial membrane potential in astrocytes was assessed through the utilization of the JC-1 Assay Kit in mPMs.
Results:
As a result of treatment with MOS, the redox status of the brain and the neuroinflammatory responses were significantly balanced. Through correlation analysis, it was observed that a modified gut microbiome and increased butyrate production had a significant impact on behavioral changes and brain oxidative status. Our findings suggest that MOS could mitigate cognitive and behavioral disorders in the Gp120 transgenic mouse. MOS achieves this by suppressing Gp120-induced chronic inflammation and dysregulated production of reactive oxygen species (ROS), thereby improving HIV-1-related neurocognitive dysfunction. This is achieved by targeting A7R and intestinal ROS signaling. Additionally, our results indicate that SCFA supplementation in the brain of HAND mice alleviated behavioral disorders and balanced the HPA-axis and redox status.
Conclusion:
Overall, the present study demonstrated that MOS significantly reduced cognitive and mental deficits in Gp120 transgenic mice. As prebiotics, MOS could be translated into novel microbiota-targeted approaches to manage metabolic and neurodegenerative diseases.
( Acknowledgements: Natural Science Foundation of Guangdong Province, No. 208059478058 to H.C.; Corresponding author: Hong Cao, gzhcao@smu.edu.cn )
Keywords:HIV-1 gp120, mangan oligosaccharide, α7nAChR, reactive oxygen species
Disclosures
No relevant conflicts of interest to declare.